Operating theatre light reduces surgical site infections
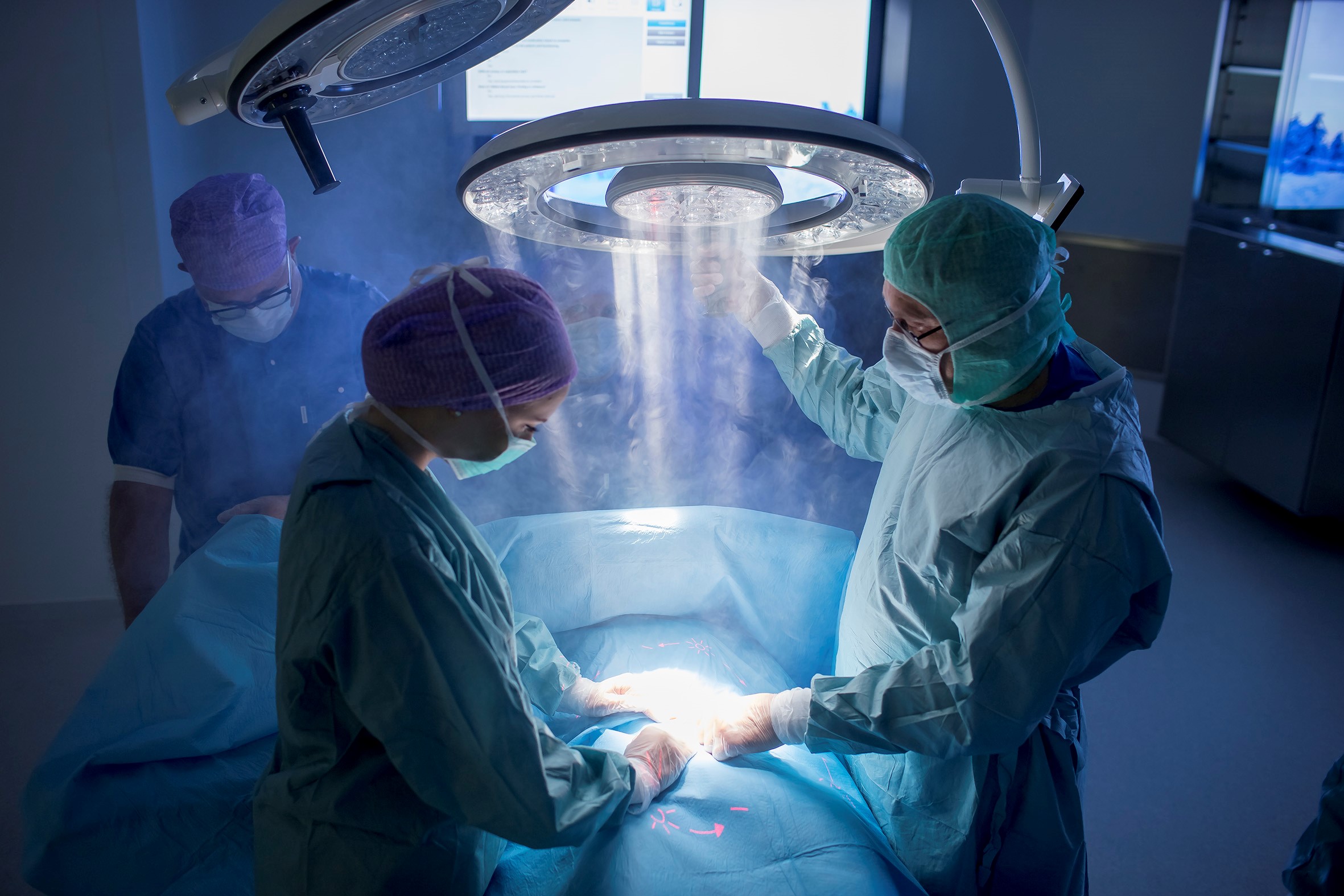
Surgical site infections constitute a huge healthcare burden worldwide and remain one of the most challenging complications to treat. Airborne particles carrying harmful microorganisms, which settle on skin and instruments, are responsible for the majority of these infections. Prevention requires an integrated approach; one that involves paying careful consideration to a variety of factors, including operating room design and air quality.
The award-winning Q-Flow™ surgical light has been uniquely designed to optimise laminar airflow and mitigate infection risks in operating theatres.
Hospital associated infections (HAIs) are a significant challenge for hospitals around the world. More than 4 million patients are estimated to acquire a healthcare-associated infection in the EU each year and approximately 1.7 million in the US are affected annually. The prevalence of HAIs in Europe is around 7.1 %1.
Surgical site Infections (SSIs) – caused by microbial contamination of surgical wounds and one of the most common causes of serious surgical complications – account for up to 16% of all healthcare-associated infections2. It is estimated that one out of every 20 patients undergoing surgery will develop an SSI3. This can be caused by a variety of patient-related (endogenous) and procedure-related (external) factors, such as age, duration of procedure, and inadequate sterilisation of instruments. The rate of surgical wound infections is strongly influenced by operating theatre quality, too4. This includes air quality, which research shows can in turn be influenced by other factors such as surgical light5. This makes the prevention of SSIs particularly complex.
Finland-based operating room equipment manufacturer Merivaara believe their award-winning surgical light provides the answer to this age-old issue. Designed and manufactured in Finland, the company’s pioneering Q-Flow™ surgical light enlists clever engineering, based on advanced research, to prevent the risk of HAIs. The new device was introduced in 2017 to respond to the strict standards of the modern operating room, with a distinctive design created with operational perfection in mind to enhance patient safety.
“Significant progress has been made in preventing healthcare-associated infections in recent years, however they still affect millions of patients worldwide, constituting a significant economic burden for health systems, increased morbidity and longer hospital stays. The surgical light Merivaara Q-Flow™ was developed to fight this issue,” Jyrki Nieminen, Director of R&D at Merivaara, explains.
“The device was designed and manufactured to provide a number of improvements on many standard features in the industry as well as radical innovations. One of the most ground-breaking features is the way it improves hygienic conditions. This is all down to the unique shape of the light, which is optimised for an operating room’s air ventilation and to minimise airflow turbulence. This decreases unwanted particles in the sterile area and reduces the infection risk.” Nieminen comments.
Preventing airborne transmission
“In today’s operating rooms, clean air enters the room through inlets in the middle of the ceiling, while dirty air is removed through outlets in the corners. By means of efficient air circulation, harmful bacteria can be kept away from patients undergoing open surgery,”
Studies show that the air of the operating theatre represents an important vehicle for contaminating micro-organisms6. During surgical procedures, airborne micro-organisms, including dust particles, skin scales and respiratory aerosols which derive mainly from people present in the operating theatre7, are released into the air. These can fall directly into the wound or on exposed surfaces, such as instruments and theatre attire, and thus be transferred into wounds causing infection. The type of procedure and instruments used can all influence the risks of transmission. Prevention relies therefore on a multidisciplinary, integrated approach.
Today, laminar airflow ventilation systems, designed to displace contaminated air away from the operational site are a common feature in many operating theatres, helping to prevent airborne transmissions. But the ventilation system is only one of a myriad of factors that can effectively contribute to a safe operating environment and it, too, can be impacted. What Merivaara hope to bring to light is the influence that surgical lights have on laminar airflow, and thus infection prevention – Nieminen explains how this revelation sits firmly at the heart of the ground-breaking design for Q-Flow™ operating theatre light:
“Managing airflow has long been a way to effectively limit infection risks during surgical procedures. However, an operating theatre is an extremely complex system, and mitigating infection risks – especially at a time when the world is battling a highly infectious virus – requires a holistic approach that considers every single aspect of the operating room."
“This is due to the nature of SSIs, which occur when harmful germs enter the body through the incision in the skin, from contaminated surfaces and instruments, and from the air. In fact, it has been suggested that the main sources of contamination in operating rooms, especially in clean surgical procedures, are the patient's skin and airborne particles from operating room personnel8.”
“It’s important to note that while airflow can be managed, it can also be disturbed,” he stresses. “The shape of lamp heads or lighting systems affect airflow. Hospitals can spend huge amounts of money on ventilation, but this is a waste if it’s not compatible with the light. Currently there is not a huge lot of research in this area; and the correlation between surgical lighting and laminar airflow is still poorly understood.
“When it came to designing Q-Flow™ therefore, the first step was understanding airflow and how exactly it works in operating rooms, and assessing the effect of different surgical lights. This has informed every stage of our development and design process."
“Effective and efficient lighting is a vital tool in patient care. Thanks to its unique design, Q-Flow™ allows air to flow through it, so that air will not circulate above the patient. Thus, it enhances the patient’s safety during operation.”
Merivaara Research and development
For Merivaara, users have been the focus of design since the beginning of 1901, when the company was founded. As early as the 1930s – back when chloroform was still a popular anaesthetic – Merivaara received several gold medals for its high-quality products, where the basic values are functionality and ease of use. Today their cutting-edge solutions continue to be developed to fulfil the needs of modern surgical teams and patients worldwide. R&D lies at the heart of their innovation strategy.
“As a manufacturer of medical device and products for healthcare, we are hugely interested in creating pioneering solutions that are used in demanding surgery applications and can improve patient safety on a global scale,” Nieminen comments.
“Our R&D team work together with doctors, surgeons, nurses and other health care specialists to develop unique innovations. Moreover, we maintain an increasing network of consultative relationships with leading academics and are on the continuous search for partnerships to inform our innovation strategy."
“Our R&D team work together with doctors, surgeons, nurses and other health care specialists to develop unique innovations. Moreover, we maintain an increasing network of consultative relationships with leading academics and are on the continuous search for partnerships to inform our innovation strategy."
“We had surgeons, anaesthesiologists and nurses give us their input as we developed Q-Flow. The unique design of the device is additionally based on research that we commissioned from an external agency, which brought to light the ways in which the shape and design of surgical lights can impact laminar airflow – and how best to limit the negative effects."
“Laminar airflow effectively equates to reduced turbulence of harmful airborne contaminants,” he explains. “Our surgical light was designed to optimise this, thus improving air flow circulation in the operating area and reducing the potential for contamination.”
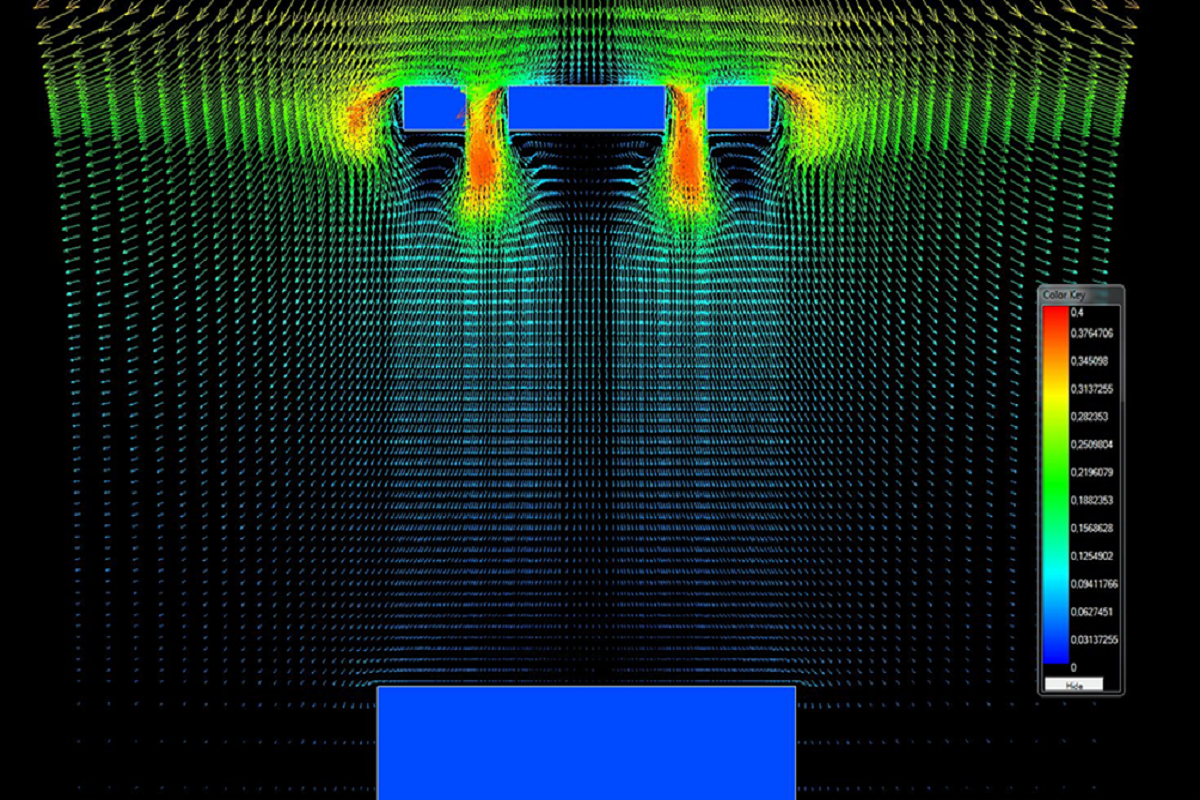
The design process
To develop the new design for QFlow™, Merivaara worked with Halton Oy to test the influence of different types of surgical lights on airflow distribution and airborne contaminations in the operating room environment.
This involved using advanced computational analysis and 3D models to simulate the air environment around the operating table to assess the impact that different surgical light shapes have on air currents and cleanliness. The results allowed them to establish the best design for reduced turbulence and thus optimal laminar airflow in the OR.
Merivaara’s designers shaped the Q-Flow™ as a series of concentric circles with open spaces in between. The distinctive design was chosen for its proven ability to reduce turbulence when compared to more standard shapes, thus helping to push more clean air towards the patient.
“This improves laminar air flow so sterile conditions are maintained and the risk of infection is reduced. The objective of laminar air flow is to deliver an uninterrupted vertical flow of sterile air to the operating table, minimising the turbulence which can introduce bacteria.”
Optimising laminar airflow
Laminar airflow systems (LAF) use positive pressure air currents to direct air streams away from the operative field and create an ultraclean zone around the operative site. The systems were pioneered by Sir John Charnley in the 1960’s and 70’s and have been shown to result in a marked decline in post-operative wound infection9 when used in conjunction with other strategies.
More recently, research has shown that the presence of surgical lights disturbs the flow of ultraclean air in operating rooms with vertical laminar airflow systems by creating a wake downstream of the lights10. This then directly influences the level of airborne microbe-carrying particles close to the surgical site, eventually leading to SSIs. An experimental study11– the first of its kind – in 2017 showed that surgical lights have a significantly negative effect on laminar airflow. Q-Flow™ provides a major step forward from traditional medical lights in response to this issue, Nieminen explains:
“Due to the optimally designed Q-Flow™, with a turbulence intensity of only 15.9 %, there is no additional particle burden created in the operating area. The device is designed for an optimised air flow that allows ventilation to work properly in an operating theatre.”
Merviaara’s Q-Flow surgical light has already won two prestigious awards for its pioneering design: the Red Dot Award for Product Design in 2017 and the Fennia Prize Grand Prix 2017 for its outstanding design.
COVID-19 and infection control
Today, growing evidence that the SARS-CoV-2 virus can be spread by aerosols12, which can hang in the air for hours and spread over distances, is placing increased significance on the importance of good ventilation, including in hospital settings.
Recent data has shown that COVID-19 might survive and be transmitted indirectly from virus contamination of common surfaces and objects after virus aerosolisation in a confined space with infected individuals13. This has particular implications for operating room environments, Nieminen concludes:
“In the absence of any viable vaccine, infection control and prevention measures are currently our best hope to contain COVID-19, especially with cases increasing across Europe. Infection control lies at the heart of surgical care – and protecting patients during this difficult time must remain a priority. In operating theatres, where the likelihood of getting infected is already disproportionately high, this must be guided by an integrated approach that can keep the risk of infection as low as possible.”
Available in UK and Ireland hospitals
Bender UK is the sole authorised distributor and maintainer of Merivaara surgical lights, operating tables, clinical trolleys, digital imaging and pendants in the UK and Republic of Ireland as part of their full turnkey healthcare offering.
Gareth Brunton, Managing Director of Bender UK, said: “The award-winning Merivaara Q-Flow surgical light is supremely designed to address one of the most prominent issues facing the modern operating theatre. The Merivaara team have invested a significant amount of time and research into developing and designing the device to ensure that it meets the needs of the patient and surgical teams worldwide. At a time when health services are operating under great strain and limited resources, it offers a unique way to reduce the burden associated with infections in the operating room. We are delighted that we can offer this cutting-edge solution to our customers in the UK and the Republic of Ireland.”
For more information about this application or to learn more about Bender technology related to your specific application, contact our team of experts.
1https://www.who.int/gpsc/country_work/gpsc_ccisc_fact_sheet_en.pdf?ua=1
2https://www.nice.org.uk/guidance/qs49/chapter/introduction
3https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4267723/
4https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4718372/
5https://www.hindawi.com/journals/jhe/2019/4861273/
6https://link.springer.com/article/10.1007/s10453-019-09584-0
7https://link.springer.com/article/10.1007/s10453-019-09584-0
8https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5139609/#R02
9https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4645891/#:~:text=Laminar%20flow%20theatres%20aim%20to,flow%20of%20bacteria%20free%20air.&text=In%20horizontal%20Laminar%20flow%20systems,of%20the%20operating%20theatre%20walls.
10https://www.sciencedirect.com/science/article/abs/pii/S0360132317304389#:~:text=The%20presence%20of%20surgical%20lights,wake%20downstream%20of%20the%20lights.
11https://online.boneandjoint.org.uk/doi/abs/10.1302/0301-620X.99B8.BJJ-2016-0581.R2
12https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7413047/
13https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5139609/#R02
For more information about this application or to learn more about Bender technology related to your specific application, contact our team of experts.
This article is for informational purposes only. Bender provides the information "as is" without warranty and is not responsible for its accuracy or reliability. No warranties are given regarding its suitability for any specific circumstances.